Recombinant antibodies offer several key advantages compared to traditional antibodies. These include superior lot-to-lot consistency, continuous supply, and amenability to antibody engineering. As such, recombinant antibodies are seeing increased use for scientific research, especially as a means of addressing the ongoing reproducibility crisis.
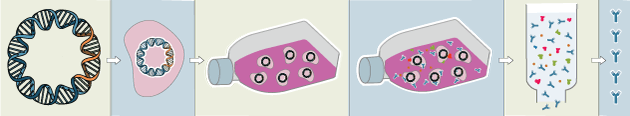
Traditional polyclonal and monoclonal antibodies are the product of normal B cell development and genetic recombination. They are generated by immunizing an animal with an antigen to elicit an immune response. While polyclonal antibodies are secreted by many different B cell clones and recognize multiple antigenic epitopes, monoclonals originate from a single B cell clone and are specific for just one epitope.
Recombinant antibodies are monoclonal, but their production involves in vitro genetic manipulation. After cloning the antibody genes into an expression vector, this is then transfected into an appropriate host cell line for antibody expression. Mammalian cell lines are most commonly used for recombinant antibody production, although cell lines of bacterial, yeast, or insect origin are also suitable.
Because recombinant antibody production involves sequencing the antibody light and heavy chains, it is a highly controlled and reliable process. In contrast, hybridoma-based systems for producing monoclonal antibodies are subject to genetic drift and instability, increasing the potential for lot-to-lot variability or loss of antibody expression. Recombinant antibodies are highly consistent from lot to lot, thereby ensuring reproducible experimental results.
In vitro methods for producing antibodies are amenable to large-scale production, meaning antibody availability is unlikely to become a limiting factor. Moreover, since the recombinant antibody sequence is known, continuity of supply is assured; in situations where an antibody will be used to support large, long-term studies, this can be an especially critical factor.
Knowing the peptide sequence of an antibody provides multiple opportunities for engineering. These include isotype-switching (also known as class-switching) and species-switching, both of which can increase the scope of multiplexing experiments by allowing the inclusion of isotype- or species-specific secondaries in panels. A further application of engineering is to improve antibody specificity using in vitro antibody selection systems such as antibody phage display.
Unlike traditional methods for antibody production, recombinant approaches avoid the need to use animals. Where polyclonal antibodies are purified directly from the serum of the immunized host, and monoclonals are purified from either hybridoma-derived tissue culture supernatant (TCS) or ascites, recombinant antibodies are instead purified from the TCS of transfected host cell lines.
Regardless of whether an antibody is polyclonal, monoclonal or recombinant, it must always be properly validated in the intended application prior to experimental use. At CST, we adhere to the Hallmarks of Antibody Validation, six complementary strategies for determining the specificity, sensitivity, and functionality of an antibody in any given assay. By carefully tailoring these strategies to each antibody product, we guarantee that CST antibodies are fit for purpose to help you achieve results you can trust.