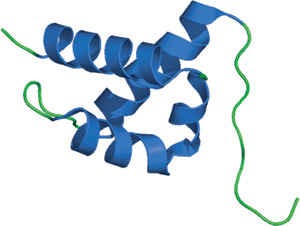
FF domain from human HYPA/FBP11.
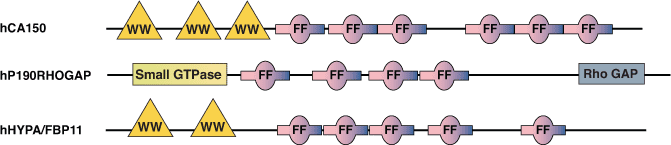
The FF domain is present in a variety of nuclear transcription and splicing factors, as well as the p190 family of RhoGAPs. The domains are well conserved from yeast to humans and are also found in plant proteins. Characterized by two conserved phenylalanines at the N- and C-termini, FF domains are ~ 50-60 amino acids in length and are often arranged in tandem repeats. FF domains from both the human transcription factor CA150 and the splicing factor hHYPA/FBP11 have been shown to interact with a serine phosphorylated C-terminal Domain (CTD) from RNA Polymerase II. FF domains from CA150 can also bind to multiple (D/E)2/5-F/W/Y-(D/E)2/5 motifs with low affinity. Interestingly, all of the nuclear FF domain-containing proteins also possess WW domains near the N-terminus. This may function in coupling the processes of transcription and splicing, as these WW domains can interact with essential splicing proteins such as mBBP/SF1.
The FF domain is composed of three α-helices arranged in an orthogonal bundle with a 310 helix positioned in a loop between the second and third helices. Consistent with its arrangement in tandem arrays, the N- and C-termini are found at opposite ends of the structure. Two highly conserved phenylalanine residues are found in the middle of the first and third helices, and help form the hydrophobic core of the protein. The FF domain structure differs significantly from other phosphoserine/threonine-binding domains, and represents a novel fold for these modules. It has been proposed that interactions with ligand might be mediated through a cluster of positively charged residues between helices 1 and 4.
| FF Domain Containing | Binding Partner |
| Human transcription factor CA150 | RNA Polymerase II C-terminal Domain (CTD) phosphorylated on serine, Tat-SF1 |
| Yeast Splicing Factor Prp40 | RNA Polymerase II C-terminal Domain (CTD) phosphorylated on serine |
| Yeast Splicing Factor HYPA/FBP11 | RNA Polymerase II C-terminal Domain (CTD) phosphorylated on serine |