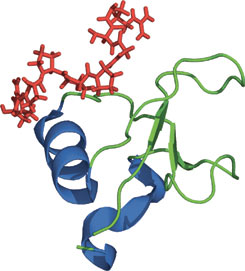
The GYF domain of CD2BP2 in complex with a proline-rich peptide of CD2.
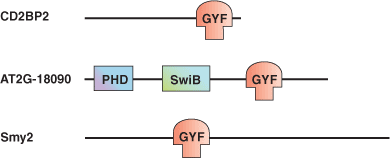
The glycine-tyrosine-phenylalanine, or GYF domain, was first reported in the CD2 binding protein CDBP2 as a domain capable of binding to a proline-rich peptide sequence in the CD2 tail region. GYF proteins are subdivided into two families: the CD2BP2-type and the SMY2-type subfamily, of which the latter is characterized by its shorter β1-β2 loop and its recognition of a PPGX motif. Despite functioning as a proline-rich peptide binding domain, the GYF fold is structurally unrelated to the SH3 or WW domains. The GYF domain of CDBP2 binds to a PPPPGHR repeat in the CD2 tail via a relatively smooth, concave surface that forms a continuous hydrophobic patch containing many of the GYF domain—conserved residues. The other class of ligands that binds to CDBP2 has the PPGW core motif.
| GYD Proteins | Binding Motif | Binding Partners |
| CD2BP2 | PPGW | CD2 |
| PERQ2 | PPGΦ | hSF1, SWAN |
| SMY2 | PPGΦ | MYO D |