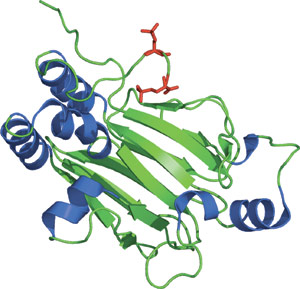
The phosphorylated (Ser465 and Ser467) Smad2 MH2 domain.
The three functional classes of Smad proteins include co-mediator Smads (co-Smads), receptor-regulated Smads (R-Smads) and inhibitory Smads (I-Smads). These Smad proteins are highly conserved within their N-terminal MH1 and C-terminal MH2 domains. The MH2 domain contains a central β-sandwich with a conserved loop-helix region that can bind phospho-serine residues. Upon ligand-receptor binding, R-Smads are recruited to the pSer-X-pSer motif on the TGFβ receptor-I where the R-Smad is phosphorylated on two Ser residues within the C-terminal SSXS motif. This allows for Smad disassociation from the receptor and heterocomplex formation with the co-Smad through its MH2 domain. In addition, phosphorylated Smad2 forms a symmetric homotrimer with both the phosphorylated C-terminus and the N-terminal extension from one MH2 domain reaching out to interact with an adjacent domain. Importantly, the pSer binding surface on the MH2 domain of Smad2 and Smad4 are frequently mutated in cancers.
| MH2 Domain Proteins | Binding Partners |
| Smad1 (R-Smad) | Type1 TGF-β receptor, BMPR-I group of receptors, ALK1 group of receptors |
| Smad2 (R-Smad) | SARA |
| Smad4 (Co-Smad) | Smad4 to form trimers by homo-oligomerization |