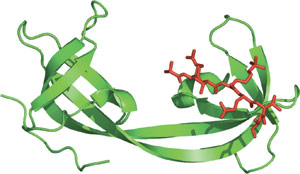
The double TUDOR domain of JMJD2A bound to a tri-methylated histone H3-K4.
The TUDOR domain was originally identified as a region of 50 amino acids found in the Drosophila TUDOR protein (a posterior group gene). The three-dimensional TUDOR domain structure of human SMN forms a strongly bent, anti-parallel β-sheet consisting of five β-strands and a barrel-like fold. The TUDOR domain was subsequently found within a number of proteins involved in RNA binding. Two TUDOR domain containing proteins, 53BP1 and JMJD2A, contain either tandem or double TUDOR domains with distinct folds. The unusual folds of these proteins are required for recognition of methylated histones. Recognition of methylated H3-K79 by 53BP1 suggests its involvement as a sensor of DNA damage in response to higher chromatin structure.
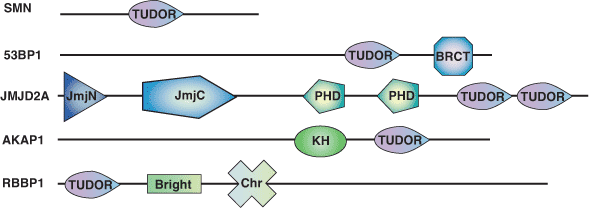
| TUDOR Domain Proteins | Binding Partners |
| 53BP1 | methylated H3-K79 |
| JMJD2A | methylated H3-K4, H4-K20 |
| SMN |