| Cat. # | Size | Qty. | Price |
|---|---|---|---|
| 11974S | 100 µl |
|
| REACTIVITY | H M R Mk |
| SENSITIVITY | Endogenous |
| MW (kDa) | 120 |
| Source/Isotype | Rabbit IgG |
Product Information
For optimal ChIP results, use 10 μl of antibody and 10 μg of chromatin (approximately 4 x 106 cells) per IP. This antibody has been validated using SimpleChIP® Enzymatic Chromatin IP Kits.
The CUT&RUN dilution was determined using CUT&RUN Assay Kit #86652.
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| Immunoprecipitation | 1:200 |
| Chromatin IP | 1:50 |
| CUT&RUN | 1:50 |
For western blots, incubate membrane with diluted primary antibody in 5% w/v BSA, 1X TBS, 0.1% Tween® 20 at 4°C with gentle shaking, overnight.
NOTE: Please refer to primary antibody product webpage for recommended antibody dilution.
From sample preparation to detection, the reagents you need for your Western Blot are now in one convenient kit: #12957 Western Blotting Application Solutions Kit
NOTE: Prepare solutions with reverse osmosis deionized (RODI) or equivalent grade water.
Load 20 µl onto SDS-PAGE gel (10 cm x 10 cm).
NOTE: Loading of prestained molecular weight markers (#59329, 10 µl/lane) to verify electrotransfer and biotinylated protein ladder (#7727, 10 µl/lane) to determine molecular weights are recommended.
NOTE: Volumes are for 10 cm x 10 cm (100 cm2) of membrane; for different sized membranes, adjust volumes accordingly.
* Avoid repeated exposure to skin.
posted June 2005
revised June 2020
Protocol Id: 10
This protocol is intended for immunoprecipitation of native proteins for analysis by western immunoblot or kinase activity utilizing Protein A magnetic separation.
NOTE: Prepare solutions with reverse osmosis deionized (RODI) or equivalent grade water.
10X Cell Lysis Buffer: (#9803) To prepare 10 ml of 1X cell lysis buffer, add 1 ml cell lysis buffer to 9 ml dH2O, mix.
NOTE: Add 1 mM PMSF (#8553) immediately prior to use.
A cell lysate pre-clearing step is highly recommended to reduce non-specific protein binding to the Protein A Magnetic beads. Pre-clear enough lysate for test samples and isotype controls.
IMPORTANT: Pre-wash #73778 magnetic beads just prior to use:
Carefully remove the buffer once the solution is clear. Add 500 μl of 1X cell lysis buffer to the magnetic bead pellet, briefly vortex to wash the beads. Place tube back in magnetic separation rack. Remove buffer once solution is clear. Repeat washing step once more.
IMPORTANT: The optimal lysate concentration will depend on the expression level of the protein of interest. A starting concentration between 250 μg/ml-1.0 mg/ml is recommended.
IMPORTANT: Appropriate isotype controls are highly recommended in order to show specific binding in your primary antibody immunoprecipitation. Use Normal Rabbit IgG #2729 for rabbit polyclonal primary antibodies, Rabbit (DA1E) mAb IgG XP® Isotype Control #3900 for rabbit monoclonal primary antibodies, Mouse (G3A1) mAb IgG1 Isotype Control #5415 for mouse monoclonal IgG1 primary antibodies, Mouse (E5Y6Q) mAb IgG2a Isotype Control #61656 for mouse monoclonal IgG2a primary antibodies, Mouse (E7Q5L) mAb IgG2b Isotype Control #53484 for mouse monoclonal IgG2b primary antibodies, and Mouse (E1D5H) mAb IgG3 Isotype Control #37988 for mouse monoclonal IgG3 primary antibodies. Isotype controls should be concentration matched and run alongside the primary antibody samples.
Proceed to one of the following specific set of steps.
NOTE: To minimize masking caused by denatured IgG heavy chains (~50 kDa), we recommend using Mouse Anti-Rabbit IgG (Light-Chain Specific) (D4W3E) mAb (#45262) or Mouse Anti-Rabbit IgG (Conformation Specific) (L27A9) mAb (#3678) (or HRP conjugate #5127). To minimize masking caused by denatured IgG light chains (~25 kDa), we recommend using Mouse Anti-Rabbit IgG (Conformation Specific) (L27A9) mAb (#3678) (or HRP conjugate #5127).
posted December 2008
revised April 2021
Protocol Id: 410
Specific for product: SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005.
Reagents Included:
Reagents Not Included:
| ! | This ! signifies an important step in the protocol regarding volume changes based on the number of immunoprecipitation preparations (IP preps). One IP prep is defined as 4 x 106 tissue cultured cells or 25 mg or disaggregated tissue. |
| !! | This !! signifies an important step to dilute a buffer before proceeding. |
| SAFE STOP | This is a safe stopping point in the protocol, if stopping is necessary. |
When harvesting tissue, remove unwanted material such as fat and necrotic material from the sample. Tissue can then be processed and cross-linked immediately, or frozen on dry ice and stored at -80°C for processing later. For optimal chromatin yield and ChIP results, use 25 mg of tissue for each immunoprecipitation to be performed. The chromatin yield does vary between tissue types and some tissues may require more than 25 mg for each immunoprecipitation. Please see Appendix A for more information regarding the expected chromatin yield for different types of tissue. One additional chromatin sample should be processed for Analysis of Chromatin Digestion and Concentration (Section IV). If desired, five additional chromatin samples should be processed for Optimization of Chromatin Digestion (Appendix B).
(!) All buffer volumes should be increased proportionally based on the number of IP preps in the experiment.
For optimal ChIP results, use approximately 4 X 106 cells for each immunoprecipitation to be performed (at least 12 X 106 cells are required in order to include positive and negative controls). For HeLa cells, one IP is equivalent to half of a 15 cm culture dish containing cells that are 90% confluent in 20 ml of growth medium. One additional sample should be processed for Analysis of Chromatin Digestion and Concentration (Section IV). Since every cell type is different, we recommend including one extra dish of cells in experiment to be used for determination of cell number using a hemocytometer or cell counter.
(!) All buffer volumes should be increased proportionally based on the number of 15 cm tissue culture dishes (or 20 ml suspension cells) used.
(!) All buffer volumes should be increased proportionally based on the number of IP preps in the experiment.
(!!) IMPORTANT: Once in solution, store 1M DTT at -20°C.
NOTE: For optimal ChIP results, it is highly critical that the chromatin is of appropriate size and concentration. Over-digestion of chromatin may diminish signal in the PCR quantification. Under-digestion of chromatin may lead to increased background signal and lower resolution. Adding too little chromatin to the IP may result in diminished signal in the PCR quantification. A protocol for optimization of chromatin digestion can be found in Appendix B.
For optimal ChIP results, use approximately 5 to 10 µg of digested, cross-linked chromatin (as determined in Section IV) per immunoprecipitation. This should be roughly equivalent to a single 100 µl IP prep from 25 mg of disaggregated tissue or 4 x 106 tissue culture cells. Typically, 100 µl of digested chromatin is diluted into 400 µl 1X ChIP Buffer prior to the addition of antibodies. However, if more than 100 µl of chromatin is required per IP, the cross-linked chromatin preparation does not need to be diluted as described below. Antibodies can be added directly to the undiluted chromatin preparation for immunoprecipitation of chromatin complexes.
(!) All buffer volumes should be increased proportionally based on the number of immunoprecipitations in the experiment.
NOTE: Most antibodies from Cell Signaling Technology work optimally between 1 and 2 ug per IP sample. In the case where there are multiple samples with varying concentrations, it is best to match the negative control Normal Rabbit IgG #2729 to the highest antibody concentration.
(!) All buffer volumes should be increased proportionally based on the number of immunoprecipitations in the experiment.
| Primer length: | 24 nucleotides |
| Optimum Tm: | 60°C |
| Optimum GC: | 50% |
| Amplicon size: | 150 to 200 bp (for standard PCR) |
| 80 to 160 bp (for real-time quantitative PCR) |
| Reagent | Volume for 1 PCR Reaction (18 µl) |
|---|---|
| Nuclease-free H2O | 12.5 µl |
| 10X PCR Buffer | 2.0 µl |
| 4 mM dNTP Mix | 1.0 µl |
| 5 µM RPL30 Primers | 2.0 µl |
| Taq DNA Polymerase | 0.5 µl |
| a. | Initial Denaturation | 95°C | 5 min |
| b. | Denature | 95°C | 30 sec |
| c. | Anneal | 62°C | 30 sec |
| d. | Extension | 72°C | 30 sec |
| e. | Repeat Steps b-d for a total of 34 cycles. | ||
| f. | Final Extension | 72°C | 5 min |
| Reagent | Volume for 1 PCR Reaction (18 µl) |
|---|---|
| Nuclease-free H2O | 6 µl |
| 5 µM RPL30 Primers | 2 µl |
| SimpleChIP® Universal qPCR Master Mix #88989 | 10 µl |
| a. | Initial Denaturation | 95°C 3 min |
| b. | Denature | 95°C 15 sec |
| c. | Anneal and Extension: | 60°C 60 sec |
| d. | Repeat steps b and c for a total of 40 cycles. | |
Analyze quantitative PCR results using the software provided with the real-time PCR machine. Alternatively, one can calculate the IP efficiency manually using the Percent Input Method and the equation shown below. With this method, signals obtained from each immunoprecipitation are expressed as a percent of the total input chromatin.
Percent Input = 2% x 2(C[T] 2%Input Sample - C[T] IP Sample)
C[T] = CT = Threshold cycle of PCR reaction
The immuno-enriched DNA samples prepared with this kit are directly compatible with ChIP-seq. For downstream NG-sequencing DNA library construction, use a DNA library preparation protocol or kit compatible with your downstream sequencing platform. For sequencing on Illumina® platforms, we recommend DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795 and its associated index primers Multiplex Oligos for Illumina® (Single Index Primers) (ChIP-seq, CUT&RUN) #29580 or Multiplex Oligos for Illumina® (Dual Index Primers) (ChIP-seq, CUT&RUN) #47538.
Recommendations:
When harvesting cross-linked chromatin from tissue samples, the yield of chromatin can vary significantly between tissue types. The table to the right provides a range for the expected yield of chromatin from 25 mg of tissue compared to 4 x 106 HeLa cells, and the expected DNA concentration, as determined in Section IV of the protocol. For each tissue type, disaggregation using a Medimachine (BD Biosciences) or a Dounce homogenizer yielded similar amounts of chromatin. However, chromatin processed from tissues disaggregated using the Medimachine typically gave higher IP efficiencies than chromatin processed from tissues disaggregated using a Dounce homogenizer. A Dounce homogenizer is strongly recommended for disaggregation of brain tissue, as the Medimachine does not adequately disaggregate brain tissue into a single-cell suspension. For optimal ChIP results, we recommend using 5 to 10 µg of digested, cross-linked chromatin per immunoprecipitation; therefore, some tissues may require harvesting more than 25 mg per each immunoprecipitation.
| Tissue/Cell | Total Chromatin Yield | Expected DNA Concentration |
|---|---|---|
| Spleen | 20-30 µg per 25 mg tissue | 200-300 µg/ml |
| Liver | 10-15 µg per 25 mg tissue | 100-150 µg/ml |
| Kidney | 8-10 µg per 25 mg tissue | 80-100 µg/ml |
| Brain | 2-5 µg per 25 mg tissue | 20-50 µg/ml |
| Heart | 2-5 µg per 25 mg tissue | 20-50 µg/ml |
| HeLa | 10-15 µg per 4 x 106 cells | 100-150 µg/ml |
Optimal conditions for the digestion of cross-linked chromatin DNA to 150-900 base pairs in length is highly dependent on the ratio of Micrococcal Nuclease to the amount of tissue or number of cells used in the digest. Below is a protocol for determination of the optimal digestion conditions for a specific tissue or cell type.
| Problem | Possible Causes | Recommendation |
|---|---|---|
| 1. Concentration of the digested chromatin is too low. | Not enough cells added to the chromatin digestion or nuclei were not completely lysed after digestion. | If DNA concentration of the chromatin preparation is close to 50 µg/ml, add additional chromatin to each IP to give at least 5 µg/IP and continue with protocol. Count a separate plate of cells before cross-linking to determine an accurate cell number and/or visualize nuclei under microscope before and after sonication to confirm complete lysis of nuclei. |
| 2. Chromatin is under-digested and fragments are too large (greater than 900 bp). | Cells may have been over cross-linked. Cross-linking for longer than 10 min may inhibit digestion of chromatin. Too many cells or not enough Micrococcal Nuclease was added to the chromatin digestion. | Perform a time course at a fixed formaldehyde concentration. Shorten the time of cross-linking to 10 min or less. Count a separate plate of cells before cross-linking to determine accurate cell number and see Appendix B for optimization of chromatin digestion. |
| 3. Chromatin is over-digested and fragments are too small (exclusively 150 bp mono-nucleosome length). Complete digestion of chromatin to mono-nucleosome length DNA may diminish signal during PCR quantification, especially for amplicons greater than 150 bp in length. | Not enough cells or too much Micrococcal Nuclease added to the chromatin digestion. | Count a separate plate of cells before cross-linking to determine accurate cell number and see Appendix B for optimization of chromatin digestion. |
| 4. No product or very little product in the input PCR reactions. | Not enough DNA added to the PCR reaction or conditions are not optimal. PCR amplified region may span nucleosome-free region. Not enough chromatin added to the IP or chromatin is over-digested. | Add more DNA to the PCR reaction or increase the number of amplification cycles. Optimize the PCR conditions for experimental primer set using purified DNA from cross-linked and digested chromatin. Design a different primer set and decrease length of amplicon to less than 150 bp (see primer design recommendations in Section VIII). For optimal ChIP results add 5-10 µg chromatin per IP. See recommendations for problems 1 and 3 above. |
| 5. No product in the positive control Histone H3-IP RPL30 PCR reaction. | Not enough chromatin or antibody added to the IP reaction or IP incubation time is too short. Incomplete elution of chromatin from Protein G beads. | Be sure to add 5-10 µg of chromatin and 10 µl of antibody to each IP reaction and incubate with antibody over-night and an additional 2 h after adding Protein G beads. Elution of chromatin from Protein G beads is optimal at 65°C with frequent mixing to keep beads suspended in solution. |
| 6. Quantity of product in the negative control Rabbit IgG-IP and positive control Histone H3-IP PCR reactions is equivalent. | Too much or not enough chromatin added to the IP reaction. Alternatively, too much antibody added to the IP reaction. Too much DNA added to the PCR reaction or too many cycles of amplification. | Add no more than 15 µg of chromatin and 10 µl of histone H3 antibody to each IP reaction. Reduce the amount of normal rabbit IgG to 1 µl per IP. Add less DNA to the PCR reaction or decrease the number of PCR cycles. It is very important that the PCR products are analyzed within the linear amplification phase of PCR. Otherwise, the differences in quantities of starting DNA can not be accurately measured. |
| 7. No product in the Experimental Antibody-IP PCR reaction. | Not enough DNA added to the PCR reaction. Not enough antibody added to the IP reaction. Antibody does not work for IP. | Add more DNA to the PCR reaction or increase the number of amplification cycles. Typically a range of 1 to 5 µg of antibody are added to the IP reaction; however, the exact amount depends greatly on the individual antibody. Increase the amount of antibody added to the IP. Find an alternate antibody source. |
posted December 2011
revised April 2022
Protocol Id: 82
| ! | This ! signifies an important step in the protocol regarding volume changes based on the number of CUT&RUN reactions being performed. |
| !! | This !! signifies an important step to dilute a buffer before proceeding. |
| SAFE STOP | This is a safe stopping point in the protocol, if stopping is necessary. |
For most cell types, live cells can be used in the CUT&RUN assay to generate robust enrichment of histones, transcription factors, and cofactors. For certain cell types that are fragile or sensitive to Conconavalin A, a light cell fixation helps to preserve the cells and keep them intact. In addition, fixation may help to boost enrichment of low abundance and/or weak binding transcription factors and cofactors if robust signal is not observed using fresh cells. Please note that over-fixation of cells will inhibit the CUT&RUN assay.
Our CUT&RUN assay works with a wide range of cell or tissue inputs. As defined in the protocol, one CUT&RUN reaction can contain between 5,000 to 250,000 cells or 1 to 5 mg of tissue. Buffer volumes used throughout the protocol do not need to be adjusted based on the amount of cells or tissue per reaction, as long as it is within this range. When indicated, buffer volumes do need to be increased proportionally based on the number of reactions being performed. If possible, we recommend using 100,000 cells or 1 mg of tissue per reaction. If cells are limiting, we recommend using at least 5,000 to 10,000 cells per reaction for histone modifications and 10,000 to 20,000 cells per reaction for transcription factors or cofactors.
NOTE: The amount of digitonin recommended for cell permeabilization is in excess and should be sufficient for permeabilization of most cell lines and tissue types. However, not all cell lines and tissues exhibit the same sensitivity to digitonin. If your specific cell line or tissue does not work with the recommended digitonin concentration, you can optimize conditions by following the protocol provided in Appendix A. Digitonin treatment should result in permeabilization of >90% of the cell population.
! All buffer volumes should be increased proportionally based on the number of CUT&RUN reactions being performed.
NOTE: Steps for live cell (no fixation) preparation should be performed in succession at room temperature to minimize stress on the cells. To minimize DNA fragmentation, avoid vigorous vortexing and cavitation of samples during resuspension. When preparing live cells for CUT&RUN, we recommend preparing the Concanavalin A Beads (Section II, Steps 1 to 5) prior to preparing the cells as to minimize the amount of time the cells sit around during bead preparation. Activated beads can be stored on ice until ready to use.
NOTE: For adherent cells, the cells first need to be detached from the dish using Trypsin and neutralized with at least 3 volumes of tissue culture medium. We do not recommend scraping the cells from the dish because this can stress and even lyse the cells. Cells should be counted using a hemocytometer or other cell counter to ensure the proper number of cells are being used for the experiment.
NOTE: The challenge of working with low cell numbers (<100,000 total cells) is that the centrifuged cell pellet is not always visible by eye, making it easy to lose cells during the wash steps. Therefore, when working with low cell numbers, we recommend skipping the wash steps 3 to 5 below. The binding of the Concanavalin A beads to cells is tolerant to having 40% cell medium in the binding reaction. Therefore, after the initial centrifugation of the cell suspension in Step 2, one can remove most of the supernatant, leaving behind ≤40 µl cell medium per reaction. Then in Step 6 add enough 1X Wash Buffer (+ spermidine + PIC) to the cell suspension to achieve a total volume of 100 µl per reaction.
NOTE: The input sample will be incubated at 55°C later in the protocol, so it is recommended to use a safe-lock 1.5 ml tube to reduce evaporation during the incubation.
NOTE: The following reagents are required for fixed cell preparation and are not included in this kit: 37% formaldehyde or 16% Formaldehyde Methanol-Free #12606, Glycine Solution (10X) #7005, and 10% SDS Solution #20533.
! All buffer volumes should be increased proportionally based on the number of CUT&RUN reactions being performed.
NOTE: With adherent cell lines, cells first need to be detached from the dish using Trypsin and neutralized with at least 3 volumes of medium. We don’t recommend scraping the cells from the dish because this can stress and even lyse the cells. Cells should be counted using a hemocytometer or other cell counter to ensure the proper number of cells are being used for the experiment.
NOTE: The challenge of working with low cell numbers (<100,000 total cells) is that the centrifuged cell pellet is not always visible by eye, making it easy to lose cells during the wash steps. In this case we do NOT recommend freezing down cell pellets. In addition, when working with these low cell numbers, we recommend skipping the wash steps 5 to 7 below. The binding of the Concanavalin A beads to cells is tolerant to having 40% cell medium in the binding reaction. Therefore, after the initial centrifugation of the cell suspension in Step 4, one can remove most of the supernatant, leaving behind ≤40 µl cell medium per reaction. Then in Step 8 add enough 1X Wash Buffer (+ spermidine + PIC) to the cell suspension to achieve a total volume of 100 µl per reaction.
NOTE: The input sample will be incubated at 55°C later in the protocol, so it is recommended to use a safe-lock 1.5 ml tube to reduce evaporation during the incubation.
For most tissue types, 1 mg of lightly fixed tissue (0.1% formaldehyde for 2 min) can generate robust enrichment of histones, transcription factors and cofactors. Formaldehyde fixation is not required for enrichment of histone modifications. However, many transcription factors and cofactors do require light fixation of the tissue for optimal results. Some low abundance and/or weak binding transcription factors and cofactors may require a medium fixation (0.1% formaldehyde for 10 min) for optimal results. In addition, medium fixation may improve results when using difficult tissue types, like fibrous tissues. Please note that over-fixation will inhibit the CUT&RUN assay. Fixed tissue samples can be frozen at -80°C for up to 6 months before using.
NOTE: When preparing fresh tissue (no fixation) for CUT&RUN, we recommend preparing the Concanavalin A Beads (Section II, Steps 1 to 5) prior to preparing the tissue as to minimize the amount of time the cells sit around during bead preparation. Activated beads can be stored on ice until ready to use.
NOTE: The following reagents are required for fixed tissue preparation and are not included in this kit: 37% formaldehyde or 16% Formaldehyde Methanol-Free #12606, Phosphate Buffered Saline (PBS) #9872, Glycine Solution (10X) #7005, and 10% SDS Solution #20533.
! All buffer volumes should be increased proportionally based on the number of CUT&RUN reactions being performed.
NOTE: For some transcription factors or cofactors, or for difficult tissue types like fibrous tissues, up to 5 mg tissue per reaction can be used without scaling up reagents.
NOTE: We recommend light fixation of tissues because this condition works optimally for most tissue types and protein targets. However, if fresh tissues are desired, skip Steps 3 to 8 and immediately proceed to Step 9.
NOTE: This volume of fixation solution is sufficient for up to 50 mg of tissue. If processing >50 mg, scale up fixation solution and 1X PBS + PIC solution in Step 7 accordingly.
NOTE: For difficult tissue types (like fibrous tissues) or low abundance and/or weak binding transcription factors or cofactors, extending the formaldehyde fixation to 10 min may improve results.
NOTE: The input sample will be incubated at 55°C later in the protocol, so it is recommended to use a safe-lock 1.5 ml tube to reduce evaporation during the incubation.
! All buffer volumes should be increased proportionally based on the number of CUT&RUN reactions being performed.
NOTE: Avoid vortexing the Concanavalin A Magnetic Bead suspension as repeated vortexing may displace the Concanavalin A from the beads.
NOTE: To avoid loss of beads, remove liquid using a pipet. Do not aspirate using a vacuum.
NOTE: If Concanavalin A Beads are prepared prior to cell or tissue preparation, as recommended for live cells and fresh tissue, the activated beads can be stored on ice until use.
NOTE: Concanavalin A Magnetic Beads may clump or stick to the sides of the tube. Beads can be resuspended by pipetting up and down.
NOTE: The amount of antibody required for CUT&RUN varies and should be determined by the user. For the positive control Tri-Methyl-Histone H3 (Lys4) (C42D8) Rabbit mAb #9751, add 2 µl of antibody to the sample. For the negative control Rabbit (DA1E) mAb IgG XP® Isotype Control (CUT&RUN) #66362, add 5 µl to the sample. We strongly recommend using the negative control antibody and NOT a no-antibody control, because the latter results in high levels of non-specific MNase digestion and high background signal. We recommend using the input sample for comparison with both qPCR and NG-seq analysis, when possible.
NOTE: Concanavalin A Magnetic Beads may clump or stick to the sides of the tube. Beads can be resuspended by pipetting up and down.
! All buffer volumes should be increased proportionally based on the number of CUT&RUN reactions being performed.
NOTE: Digitonin Solution #16359 should be stored at -20°C. Please keep on ice during use and store at -20°C when finished for the day.
NOTE: Concanavalin A Magnetic Beads may clump or stick to the sides of the tube. Beads can be resuspended by pipetting up and down.
! All buffer volumes should be increased proportionally based on the number of CUT&RUN reactions being performed.
NOTE: Digitonin Solution #16359 should be stored at -20°C. Please keep on ice during use and store at -20°C when finished for the day.
Optional: Sample Normalization Spike-In DNA can be added into the 1X Stop Buffer if sample normalization is desired (for example, see Figure 8 in Section VII). For qPCR analysis, we recommend adding 5 µl (5 ng) of Spike-In DNA to each reaction. For NG-seq analysis, we recommend diluting the Sample Normalization Spike-In DNA 100-fold into Nuclease-free Water #12931 and then adding 5 µl (50 pg) of Spike-In DNA to each reaction. When using 100,000 cells or 1 mg of tissue per reaction this ensures that the normalization reads are around 0.5% of the total sequencing reads. If more or less than 100,000 cells or 1 mg of tissue are used per reaction, proportionally scale the volume of Sample Normalization Spike-In DNA up or down to adjust normalization reads to around 0.5% of total reads.
NOTE: Digestion should be performed in a 4°C cooling block or refrigerator. The temperature of ice can get as low as 0°C, which can limit digestion and decrease signal.
NOTE: If live cells or fresh tissues (not fixed) are used for the CUT&RUN assay, skip Steps 14-15 and immediately proceed to Step 16.
NOTE: Fixed samples will be incubated at 65°C later in the protocol, so it is recommended to use a safe-lock 2 ml tube to reduce evaporation during the incubation.
NOTE: SDS may precipitate out of solution if samples are not pre-warmed to room temperature.
Fragmentation of input DNA is required for compatibility with downstream NG-Sequencing but is not necessary for downstream qPCR analysis. If a sonicator is not available, we recommend using the unfragmented input DNA for qPCR analysis; however, the input DNA should be purified using phenol/chloroform extraction and ethanol precipitation because the size of unfragmented input DNA is too large to be purified using DNA spin columns. If a sonicator is not available and downstream NG-Sequencing analysis is desired, one can use the CUT&RUN normal IgG antibody sample as the negative control, although this is not ideal because the normal IgG-enriched sample may show non-specific DNA enrichment. Alternatively, an input DNA fragmentation protocol using MNase is available at https://cst-science.com/CUT-RUN-input-digestion.
! All buffer volumes should be increased proportionally based on the number of input samples being prepared.
NOTE: Sonication conditions may need to be determined empirically by testing different sonicator power settings and/or durations of sonication, following the protocol in Appendix B. Optimal sonication conditions will generate chromatin fragments ranging in size from 100-600 bp. Sonication for 5 sets of 15-sec pulses using a VirTis Virsonic 100 Ultrasonic Homogenizer/Sonicator at setting 6 with a 1/8-inch probe sufficiently fragments the input chromatin.
DNA can be purified from input and enriched chromatin samples using DNA spin columns, as described in Section VI - A, or phenol/chloroform extraction followed by ethanol precipitation as described in Section VI - B. Purification using DNA spin columns is simple and fast, providing good recovery of DNA fragments above 35 bp (Figure 7A, Lane 2). Phenol/chloroform extraction followed by ethanol precipitation is more difficult, but provides better recovery of DNA fragments below 35 bp (Figure 7A, Lane 3); however, as shown in Figure 7B, the majority of DNA fragments generated in the CUT&RUN assay are larger than 35 bp. Therefore, DNA spin columns provide a quick and simple method for purification of > 98% of the total CUT&RUN DNA fragments.
Purified DNA can be quantified prior to NG-seq analysis using a picogreen-based DNA quantification assay. For CUT&RUN reactions containing 100,000 cells, the expected DNA yield for a CUT&RUN reaction ranges from 0.5 to 10 ng per reaction for transcription factors and cofactors, and 1 to 20 ng per reaction for histone modifications.
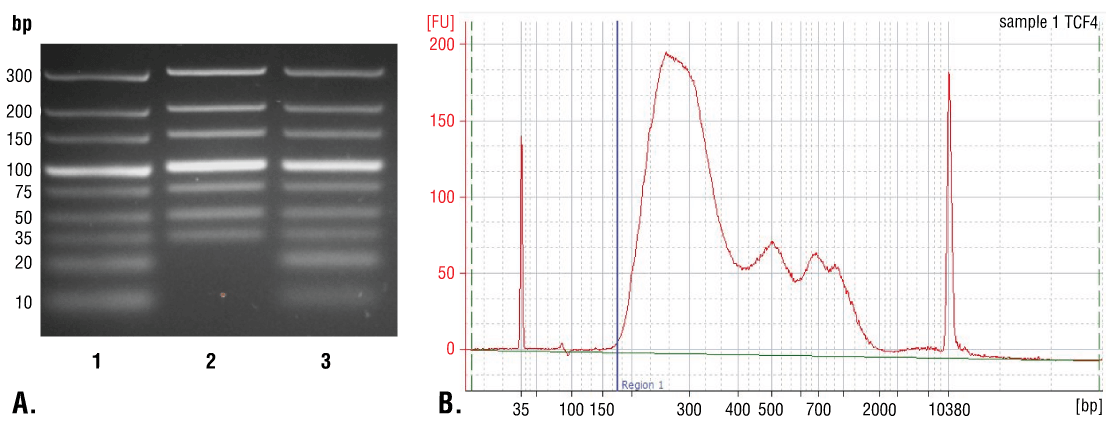
FIGURE 7. Comparison of DNA purification using spin columns or phenol/chloroform extraction followed by ethanol precipitation. (A) A low range DNA ladder mix (lane 1, unpurified) was purified using either DNA Purification Buffers and Spin Columns (ChIP, CUT&RUN) #14209 (lane 2) or phenol/chloroform extraction followed by ethanol precipitation (lane 3) and separated by electrophoresis on a 4% agarose gel. As shown, phenol/chloroform followed by ethanol precipitation efficiently recovers all DNA fragment sizes, while DNA spin columns recover DNA fragments ≥ 35 bp. (B) DNA was purified using phenol/chloroform extraction followed by ethanol precipitation from a CUT&RUN assay performed using TCF4/TCF7L2 (C48H11) Rabbit mAb #2569. The size of the DNA fragments in the library was analyzed using a Bioanalyzer (Agilent Technologies). The adaptor and barcode sequences added to the library during construction account for 140 bp in fragment length. Therefore, starting 35 bp DNA fragments would be 175 bp in length after library preparation (indicated with blue vertical line in figure). As shown, less than 2% of the total CUT&RUN enriched DNA fragments are less than 175 bp (starting length of 35 bp), suggesting that DNA purification spin columns are sufficient for capture of > 98% of the total CUT&RUN DNA fragments.
NOTE: DNA can be purified from input and enriched chromatin samples using the DNA Purification Buffers and Spin Columns (ChIP, CUT&RUN) #14209 (not included in this kit) and the modified protocol below. Steps 1 through 5 are modified to reflect the requirement for adding 5 volumes (1.5 ml) of DNA Binding Buffer to the 300 µl of input and enriched chromatin samples.
NOTE: 5 volumes of DNA Binding Buffer should be used for every 1 volume of sample.
NOTE: The following reagents are required for the phenol/chloroform extraction and ethanol precipitation and are not included in this kit: phenol/chloroform/isoamyl alcohol (25:24:1), chloroform/isoamyl alcohol (24:1), 3M Sodium Acetate (pH 5.2), 20mg/ml glycogen, 100% ethanol, 70% ethanol, and 1X TE buffer or Nuclease-free Water #12931.
NOTE: If sample normalization is performed, only the CUT&RUN samples are to be analyzed using the Sample Normalization Primer Set. The input DNA does not contain the Normalization Spike-In DNA.
| Reagent | Volume for 1 PCR Reaction (18 µl) |
|---|---|
| Nuclease-free H2O #12931 | 6 µl |
| 5 µM Primers | 2 µl |
| SimpleChIP® Universal qPCR Master Mix #88989 | 10 µl |
| a. | Initial Denaturation | 95°C for 3 min |
| b. | Denature | 95°C for 15 sec |
| c. | Anneal and Extension | 60°C for 60 sec |
| d. | Repeat steps b and c for a total of 40 cycles. |
| C[T] value of Sample Normalization Primer Set | **Normalization Factor for qPCR | Signal Before Normalization (% Input Calc'd from Step 5) | Signal After Normalization | |
| Sample 1 | 23.31 | 2(23.31-23.31)=1.00 | 24.4% | 24.4%/1.00=24.4% |
| Sample 2 | 24.24 | 2(23.31-24.24)=0.52 | 12.0% | 12.0%/0.52=23.1% |
| Sample 3 | 25.08 | 2(23.31-25.08)=0.29 | 6.28% | 6.28%/0.29=21.7% |
| Sample 4 | 26.30 | 2(23.31-26.30)=0.13 | 2.72% | 2.72%/0.13=20.9% |
**Normalization Factor for qPCR = 2(C[T] Selected Sample - C[T] the Other Sample)
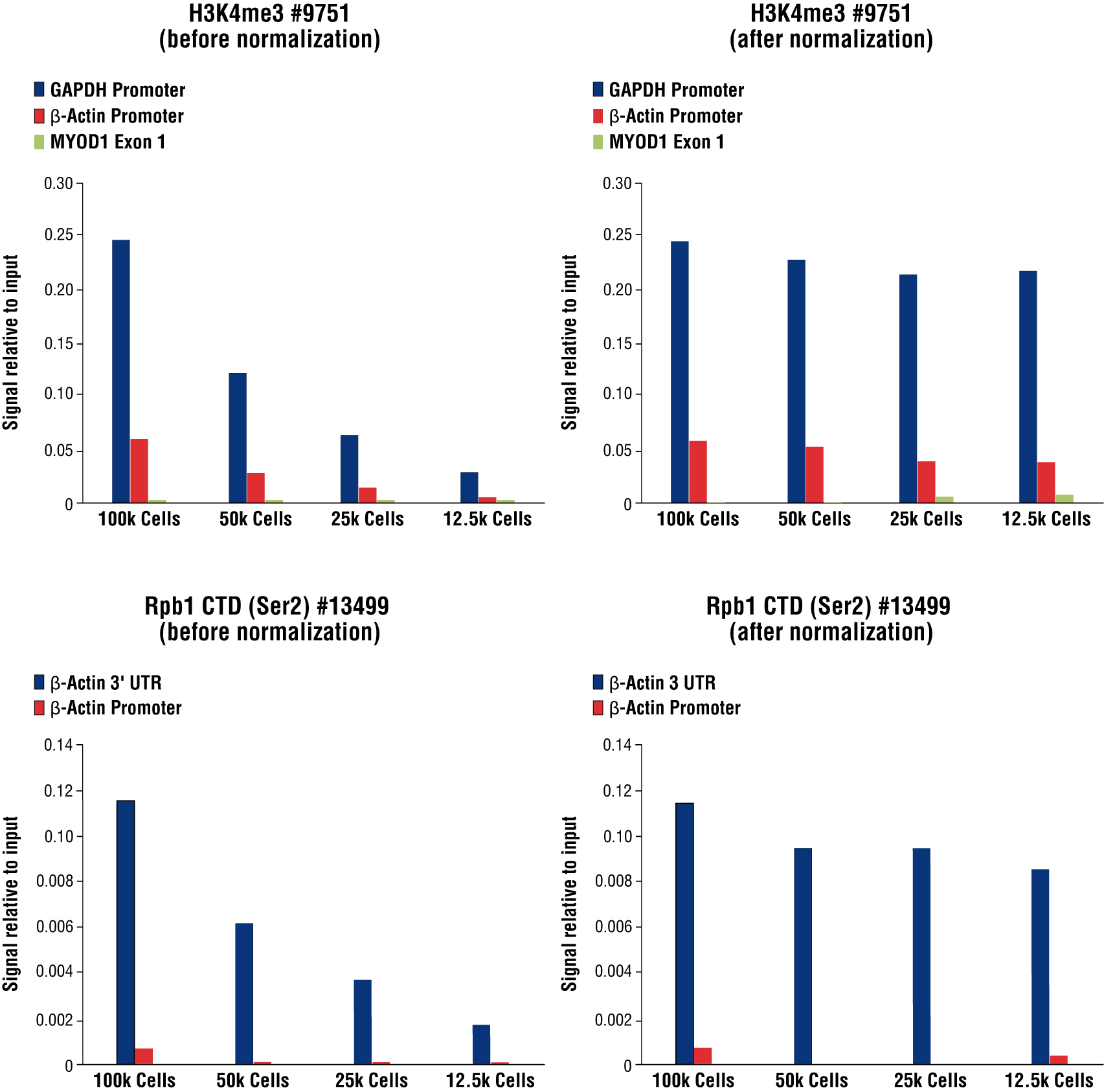
FIGURE 8. Normalization of CUT&RUN signals using spike in DNA for qPCR analysis. CUT&RUN was performed with a decreasing number of HCT116 cells and either Tri-Methyl-Histone H3 (Lys4) (C42D8) Rabbit mAb #9751 (upper panels) or Phospho-Rpb1 CTD (Ser2) (E1Z3G) Rabbit mAb #13499 (lower panels). Enriched DNA was quantified by real-time PCR using SimpleChIP® Human GAPDH Exon 1 Primers #5516, SimpleChIP® Human β-Actin Promoter Primers #13653, SimpleChIP® Human β-Actin 3' UTR Primers #13669, and SimpleChIP® Human MyoD1 Exon 1 Primers #4490. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin for 100,000 cells. Non-normalized enrichments are depicted in the left panels. The Sample Normalization Spike-In DNA was added into each reaction proportionally to the starting cell number. Based on the difference of qPCR signals from spike in DNA in each sample, CUT&RUN signals were normalized to the sample containing 100,000 cells. Normalized enrichments are depicted in the right panels.
The immuno-enriched DNA samples prepared with this kit are directly compatible with NG-seq. For downstream NG-seq DNA library construction, use a DNA library preparation protocol or kit compatible with your downstream sequencing platform. For sequencing on Illumina® platforms, we recommend using DNA Library Prep Kit for Illumina® (ChIP-seq, CUT&RUN) #56795 with Multiplex Oligos for Illumina® (ChIP-seq, CUT&RUN) #29580 or #47538, following the Protocol for CUT&RUN DNA.
| The Number of Unique Reads Aligned to Yeast | Normalization Factor for NGS | The Number of Unique Reads Aligned to Test Reference Genome Before Normalization | The Number of Unique Reads Aligned to Test Reference Genome After Normalization | |
| Sample 1 | 219,275 | 219,275/219,275 = 1.00 | 5,077,747 | 5,077,747 X 1.00 = 5,077,747 |
| Sample 2 | 411,915 | 219,275/411,915 = 0.53 | 9,896,671 | 9,896,671 X 0.53 = 5,268,306 |
| Sample 3 | 816,235 | 219,275/816,235 = 0.27 | 17,842,773 | 17,842,773 X 0.27 = 4,793,320 |
| Sample 4 | 1,120,826 | 219,275/1,120,826 = 0.20 | 23,836,679 | 23,836,679 X 0.20 = 4,663,339 |
Normalization Factor for NGS = the number of unique yeast reads from Selected Sample / the number of unique yeast reads from the other sample
In the CUT&RUN protocol, the addition of digitonin to the buffers facilitates the permeabilization of cell membranes and entry of the primary antibody and pAG-MNase enzyme into the cells and nuclei. Therefore, having an adequate amount of digitonin in the buffers is critical to the success of antibody and enzyme binding and digestion of targeted genomic loci. Different cell lines show differing sensitivities to digitonin cell permeabilization. While the amount of digitonin recommended in this protocol should be sufficient for permeabilization of most cell lines or tissues, you can test your specific cell line or tissue using this protocol. We have found that the addition of excess digitonin is not deleterious to the assay, so there is no need to perform a concentration curve. Rather, a quick test to determine if the recommended amount of digitonin works for your cell line is sufficient.
NOTE: Digitonin Solution #16359 should be stored at -20°C. Please keep on ice during use and store at -20°C when finished for the day.
NOTE: If the cell pellet is not visible by eye, we recommend removing as much cell medium as possible without disturbing the cell pellet after the initial centrifugation of the cell suspension in Step 2 and leave behind some cell medium per reaction. Then in Step 3 add enough 1X Wash Buffer to the cell suspension to achieve a total volume of 100 µl.
Sonication of the input DNA sample is recommended because only fragmented genomic DNA (<10 kb) can be purified using DNA purification spin columns. Additionally, the fragmented genomic DNA (<1kb) may be used as the negative control in NG-seq analysis. Sonication should be optimized so that the input DNA is 100-600 bp in length.
We recommend using the input sample for NG-seq because it provides a convenient and unbiased representation of the cell genome. While the IgG sample can also be used as a negative control for NG-seq, it may show enrichment of specific regions of the genome due to non-specific binding. Unfragmented input DNA can be used for qPCR analysis. However, unfragmented DNA must be purified using phenol/chloroform extraction followed by ethanol precipitation.
! All buffer volumes should be increased proportionally based on the number of input samples being prepared.
NOTE: If the centrifuged cell pellet is not visible by eye when working with low cell numbers (<100,000 cells), we recommend skipping the wash steps 3-5 below. Remove as much cell medium as possible without disturbing the cell pellet after the initial centrifugation of the cell suspension in Step 2 and leave behind some cell medium per reaction. Then in Step 6 add enough 1X Wash Buffer to the cell suspension to achieve a volume of 100 µl per sonication condition being tested.
NOTE: Samples will be incubated at 55°C in Step 9, so it is recommended to use a safe-lock 1.5 ml tube to reduce evaporation during the incubation.
For a detailed troubleshooting guide, please go to https://cst-science.com/troubleshooting-CUT-RUN
Protocol Id: 1884
Human, Mouse, Rat, Monkey
Hamster, Dog, Pig, Horse, Guinea Pig
Monoclonal antibody is produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Gly516 of human RNF20 protein.
In mammalian cells, the significance of histone H2B ubiquitination in chromatin epigenetics came from the identification of the budding yeast protein Bre1 (1,2). Together with the ubiquitin-conjugating enzyme Rad6, Bre1 serves as the E3 ligase in the monoubiquitination of the yeast histone H2B within transcribed regions of chromatin (1-3). Subsequently, the mammalian orthologs of yeast Bre1, RNF20 and RNF40, were identified (4,5). These two proteins form a tight heterodimer that acts as the major E3 ligase responsible for histone H2B monoubiquitination at Lys120 in mammalian cells, a modification linked to RNA Pol II-dependent transcription elongation in undamaged cells. Researchers have shown that DNA double-strand breaks (DSBs) are also capable of inducing monoubiquitination of H2B. This process depends upon the recruitment to DSB sites, as well as ATM-dependent phosphorylation of the RNF20-RNF40 heterodimer, thus highlighting a role for this E3 ligase in DSB repair pathways (6). Indeed, investigators have shown that loss of RNF20-RNF40 function promotes replication stress and chromosomal instability, which may constitute an early step in malignant transformation that precedes cell invasion (7).
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.