On a global scale, Alzheimer’s disease (AD) is the most common neurodegenerative disease. The clinical presentation includes the presence of intracellular neurofibrillary tangles and extracellular amyloid plaques that lead to neuronal dysfunction and cell death.
Amyloid Precursor Protein (APP) is a 100-140 kDa transmembrane glycoprotein that plays a major role in the pathogenesis of AD. In healthy individuals, APP cleavage by α-secretase generates a C83 carboxy-terminal fragment and soluble APP, which is associated with normal synaptic transmission.
In the diseased state, APP is abnormally cleaved first by β-secretase and then γ-secretase. This releases the amyloid beta (Aβ) peptides Aβ40 and Aβ42, neurotoxic fragments capable of oligomerizing, aggregation, and subsequent plaque formation. The buildup of Aβ40/42 inhibits ion channels, impedes calcium homeostasis, and impairs neuronal energy metabolism, eventually leading to neuronal cell death.
In addition, proteolytic processing and secretion APP can be affected by phosphorylation.
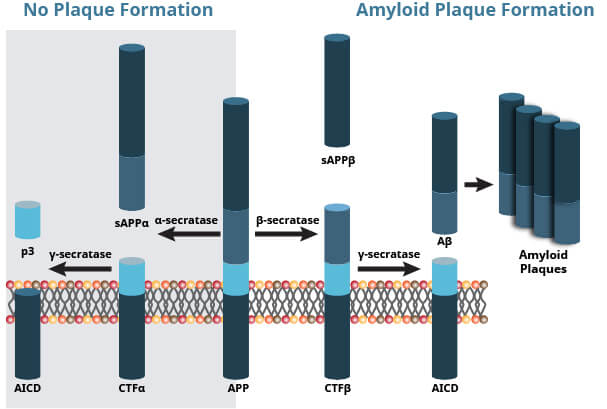
APP Processing by Secretase Enzymes
Three hallmarks of AD include:
Abnormal processing of APP and the release of the neurotoxic Aβ fragments as described above results first in the aggregation of Aβ into oligomers that subsequently group together to form fibrils. Next, the fibrils cluster together to form amyloid plaques. These plaques prevent synaptic transmission, activate inflammatory responses, and disrupt neuronal metabolism, which all contribute to the death of the neuron.
To determine the physiological and pathogenic function of APP and Aβ in AD, it is important to have a method of accurate and definitive measurement for these proteins.
The solubility and quantity of Aβ has been implicated in the differential presentation of AD. While APP is in high abundance, Aβ is found in low abundance (picomolar) quantities as measured in human cerebro-spinal fluid. Traditional approaches to determine expression levels of APP and Aβ have been through immunohistochemistry, qPCR, and ELISA.
The detection of Aβ in plasma has been challenging, although progress has been made through mass spectrometry and other methods. Detecting changes to levels of Aβ peptides could be beneficial to making an early diagnosis of AD, and potentially resulting in a more positive clinical outcome.
Recent research has suggested that accumulation of Aβ results in phagocytosis and clearance by microglia. In addition, microglia are known to tightly pack the aggregated Aβ, which prevents the addition of new Aβ to plaques, and in turn, protects neurons from degeneration in a TREM2- and apoE-dependent manner.
As a therapeutic, pharmaceutical companies are driven towards creating novel treatments to lessen the levels of Aβ in the brain. By assisting in the clearance of plaques, anti-amyloid immunotherapies represent a potential direction in the treatment of AD.