Cell Signaling Technology® (CST®) offers custom synthesized, isotope-labeled AQUA peptides for validating and quantifying protein markers and post-translational modifications. AQUA peptides enable classic isotope dilution strategies – the gold standard of quantitative mass spectrometry – for the analysis of protein and post-translational modification markers. We specialize in high-quality custom AQUA peptide synthesis, including challenging sequences and unusual post-translational modifications. We have many hundreds of synthetic AQUA peptides in stock, and turn-around on new AQUA peptide synthesis and quality control is approximately 4 – 6 weeks.
Each synthetic AQUA peptide is designed to be identical in sequence to cellular peptides generated by protease digestion of a protein extract and to contain at least one amino acid residue with the stable isotopes C13 and N15 in place of C12 and N14. AQUA peptides have the same properties as their biologically derived counterparts except their masses are slightly higher. When added to a biological sample, AQUA peptides will fractionate with their corresponding endogenous partner peptides. Only in the mass spectrometer will the mass difference between the endogenous (“light”) peptide and synthetic AQUA (“heavy”) peptide result in separation.
CST scientists helped launch the AQUA peptide technology (Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP (2003) Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. U.S.A. 100(12), 6940–5.). We and many other researchers use AQUA peptides routinely to confirm and validate novel post-translational modification sites. The co-elution in liquid chromatography (LC) of an AQUA peptide standard and a biological peptide is compelling evidence that the biological peptide sequence, including the location of post-translational modification sites, has been assigned correctly. In addition, AQUA peptide LC peak area provides a means of quantitatively measuring the absolute or relative amounts of an endogenous peptide and post-translational modification sites.
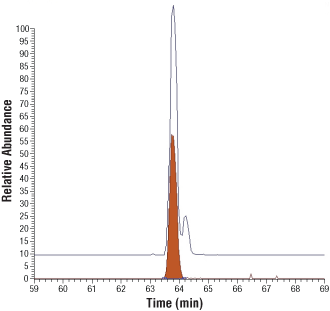
AQUA peptides as identity standards for post-translational modifications. Upper trace, extracted ion chromatogram for tryptic peptides from the activation loop of Met protein kinase, corresponding to phosphorylation of Tyr-1234 (earlier eluting peak) and Tyr-1235 (later eluting peak). Lower trace, extracted ion chromatogram for the Tyr-1234 AQUA peptide. Co-elution confirms the earlier eluting peak is the peptide phosphorylated at Tyr-1234.
Cell Signaling Technology Sales:
phone: 877-616-2355
Contact Sales Department
Purchase of this peptide product includes a license to use this product in the practice of methods described in United States Patent Application Ser. No. 60/312,279 and PCT US02/25778 (Gygi et al., Harvard University; PROTEIN-AQUA™ methodology/technology) for research purposes only, and the purchase price includes an allowance for payment to Harvard University of its standard royalty for such license. Licenses for the practice of the methods for other non-research purposes may be sought directly from the owner of the technology:
Harvard University
Office of Technology Licensing
Building A, Room 414
25 Shattuck Street
Boston, MA 02115
The purchase of this peptide product does not include a license to use this product in the practice of methods described in United States Patent Application Ser. No. 60/312,279 and PCT US02/25778 (Gygi et al., Harvard University; PROTEIN-AQUA™ methodology/technology). Licenses for the practice of such methods must be sought directly from the owner of the technology:
Harvard University
Office of Technology Licensing
Building A, Room 414
25 Shattuck Street
Boston, MA 02115