Immunostaining uses antibodies to detect an antigen in cells or tissue. The major benefit of immunostaining in immunohistochemistry (IHC) is the ability to see the desired target in a tissue sample while maintaining the spatial context and tissue architecture.
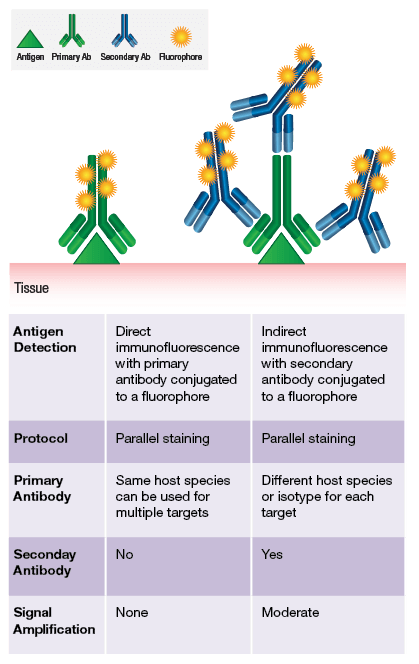
Immunostaining can be performed either directly or indirectly. Conjugating our application-validated antibodies to a number of fluorophores enables direct labeling. To browse our offering of direct conjugates, click here.
Often based on antigen expression levels and accessibility, a direct conjugate is not sufficient to visualize the target of interest. In these cases, indirect labeling is employed using a secondary antibody. The preferred method at CST is to utilize highly sensitive, one-step, polymer-based detection reagents specific for rabbit or mouse IgG.
Some examples of IHC staining performed at CST are shown below. Each of the antibodies used below have undergone our rigorous validation process in order to confidently assess specific detection of the desired antigen.
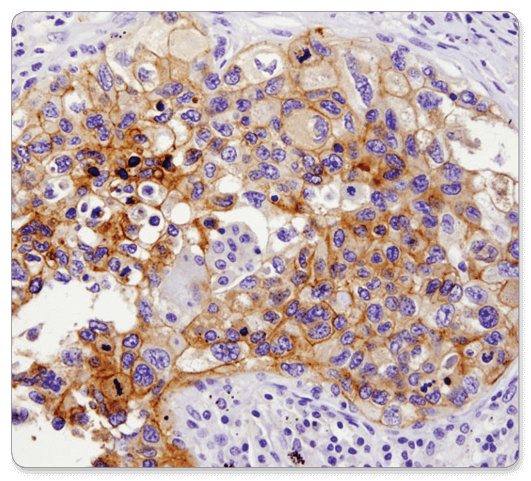
PD-L1 (E1L3N®) XP® Rabbit mAb #13684: IHC analysis of paraffin-embedded human lung carcinoma using PD-L1 (E1L3N®) XP® Rabbit mAb.
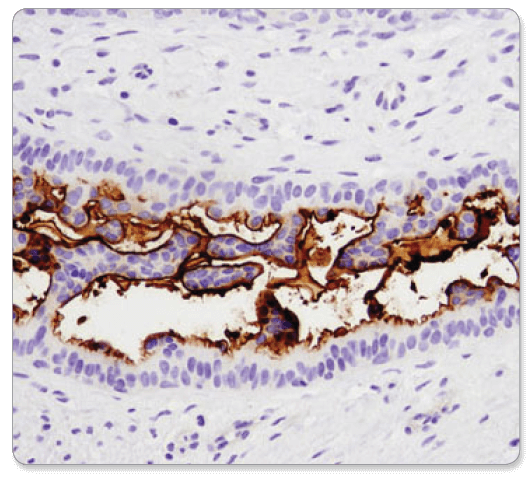
CD133 (D2V8Q) XP® Rabbit mAb #64326: IHC analysis of paraffin-embedded human breast carcinoma using CD133 (D2V8Q) XP® Rabbit mAb.
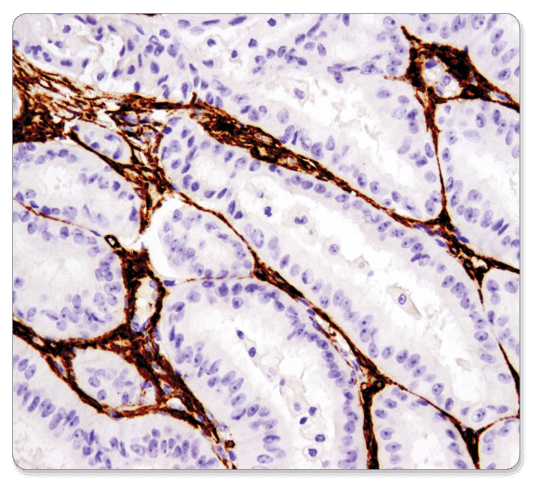
α-Smooth Muscle Actin (D4K9N) XP® Rabbit mAb #19245: IHC analysis of paraffin-embedded human endometrioid carcinoma using α-Smooth Muscle Actin (D4K9N) XP® Rabbit mAb performed on the Leica® Bond™ Rx.
Tissue collection, preservation, and fixation procedures vary depending on the sample or the target of interest. Collection and fixation of the tissue directly affects sample integrity and your experimental results.
Cross-linking fixatives, like formalin, are often used to prepare samples for IHC because they preserve the structural integrity of the tissue, which helps maintain tissue architecture. Another option is to cryopreserve (freeze) your samples, which may be a better option if you are attempting to detect a phosphorylated target.
Fixatives used to preserve the structural integrity of the tissue sample also have drawbacks in that they may bury the epitope your antibody is designed to recognize.
Several methods exist for revealing epitopes that have been masked by fixation. These include proteolytic-induced antigen retrieval, which relies on an enzyme like proteinase K, or heat-induced epitope retrieval (HIER), which uses heat to break apart cross-linked bonds and unwind proteins. Either method can unmask epitopes, rendering them accessible to the primary antibody and amenable to staining by IHC.
At CST, our most common antigen retrieval method is HIER. However, our scientists test multiple methods of antigen retrieval to determine the optimal conditions for use of each antibody in IHC. The recommended protocol from CST can be found on the datasheet of any IHC-validated antibody.
Selecting the right antibody is one of the most critical steps in obtaining a successful IHC result. All IHC-validated antibodies from CST are verified not only as having a strong signal in tissue but undergo a stringent validation procedure to confirm specificity of the signal. Our scientists, specializing in IHC, test a large number of antibodies and only recommend those best suited for the application.
Click here to browse IHC-validated antibodies for your target.
Immunohistochemistry (IHC) staining allows for 2 broad classes of detection: 1) chromogenic and 2) fluorescent. For chromogenic detection, CST recommends using polymer-based systems that avoid the limitations of the biotin-based system while also increasing sensitivity of the assay.
Try SignalStain® Boost IHC Detection Reagent (HRP, rabbit) #8114 or SignalStain® Boost IHC Detection Reagent (HRP, mouse) #8125 depending on the host species of your primary antibody.
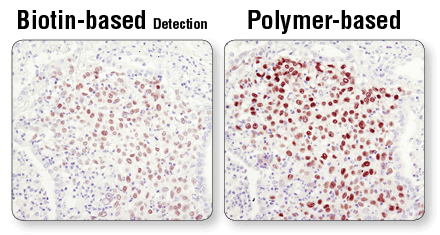
Polymer-based detection is more sensitive than biotin-based systems. IHC analysis of paraffin-embedded human lung carcinoma using Sox2 (D6D9) XP® Rabbit mAb #3579 and either biotin-based detection (left) or polymer-based detection (SignalStain® Boost IHC Detection Reagent #8114; right). As shown, polymer-based detection offers enhanced sensitivity and results in more robust staining.

Not all DAB substrate performs equally. IHC analysis of paraffin-embedded human breast carcinoma using Phospho-Stat3 (Tyr705) (D3A7) XP® Rabbit mAb #9145. Chromogenic detection was performed using SignalStain® DAB Substrate Kit #8059 (left), DAB supplied by competitor 1 (middle), or DAB supplied by competitor 2 (right). Although competitor 2 DAB produces a signal comparable to the SignalStain® DAB Substrate Kit, competitor 1 DAB results in a much weaker signal.
After antibody detection, many researchers prefer to counterstain the tissue to visualize cellular anatomy and orient the viewer with respect to the specific staining. At CST, we counterstain our samples using Hematoxylin #14166, which colors the nucleus blue. When choosing a counterstain, it is important that the counterstain be compatible with your chromogen. For example, if the counterstain color is too similar to the chromogen color, the antibody signal will be difficult to recognize.
Below is an overview of our recommended protocol and highlighted are the key steps to a successful experiment. CST aims to help you improve your IHC analysis by providing validated antibodies and reagents to allow you to follow a simple, product-specific protocol and achieve reproducible results with minimal optimization required.
Watch the IHC Protocol Video or visit our protocol selection page.
Link #9 to: https://www.cellsignal.com/contents/resources/protocols/resources-protocols -->